Sozinibercept demonstrated superior visual outcomes in wet AMD Phase 2b clinical trial
In a large prospective, randomized and controlled Phase 2b clinical trial of 366 treatment-naïve wet AMD patients, sozinibercept demonstrated superior vision outcomes in combination with standard-of-care ranibizumab compared to ranibizumab alone.
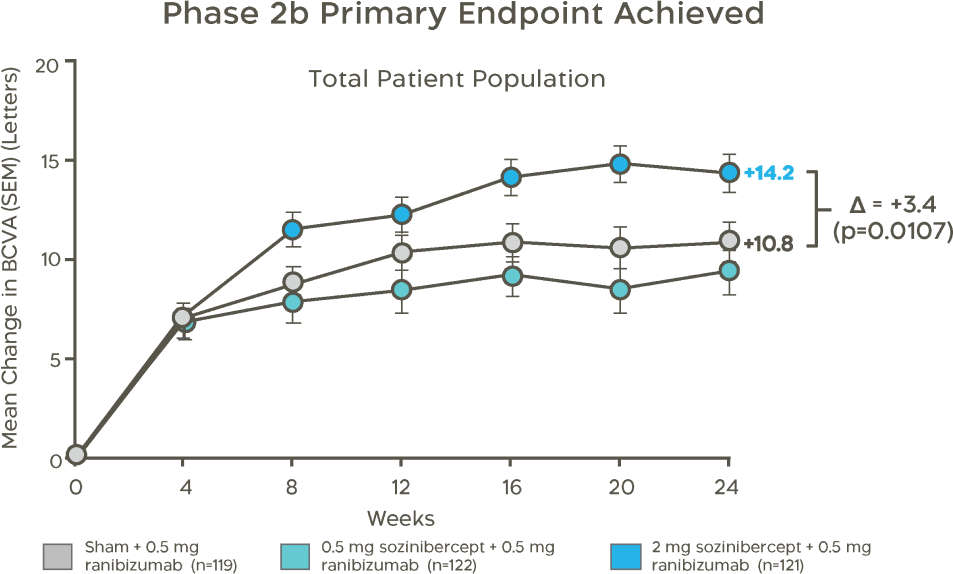
Sozinibercept combination therapy met the primary efficacy endpoint of a statistically significant superior gain in visual acuity of +3.4 letters at 24 weeks, compared to ranibizumab alone.
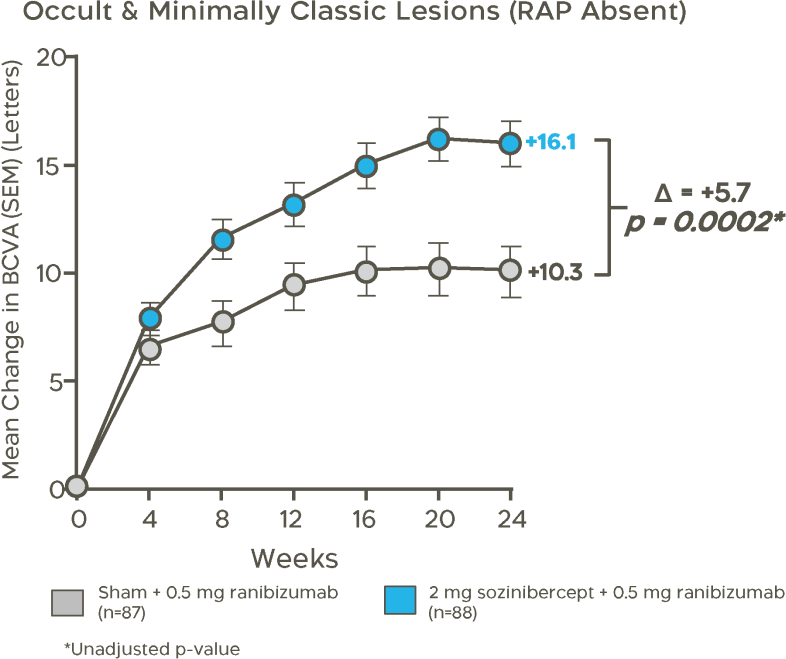
In a pre-specified population of patients that represents 73% of wet AMD patients with occult and minimally classic lesions, RAP absent, sozinibercept combination therapy demonstrated a statistically significant superior gain in visual acuity of +5.7 letters at 24 weeks compared to standard of care alone.
With sozinibercept combination therapy, more patients gained 15 letters and had improvements in all retinal anatomy parameters, i.e. reduction in swelling and vascular leakage, lesion size, fluid in the retina. Sozinibercept combination therapy had a favorable safety profile.
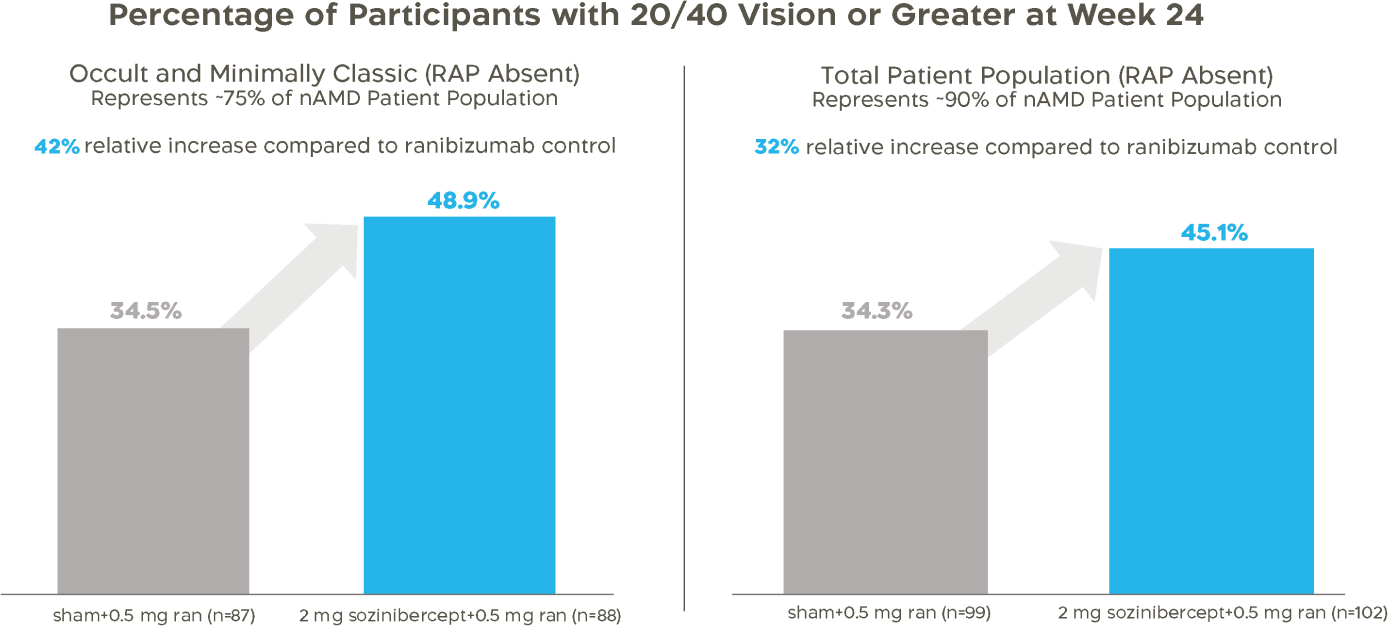
Sozinibercept Phase 2b data show greater proportion of patients who received sozinibercept combination therapy achieved minimum driving-level vision and fewer patients experienced vision loss.
A significant proportion of patients who received sozinibercept combination therapy achieved minimum driving-level vision (≥20/40).
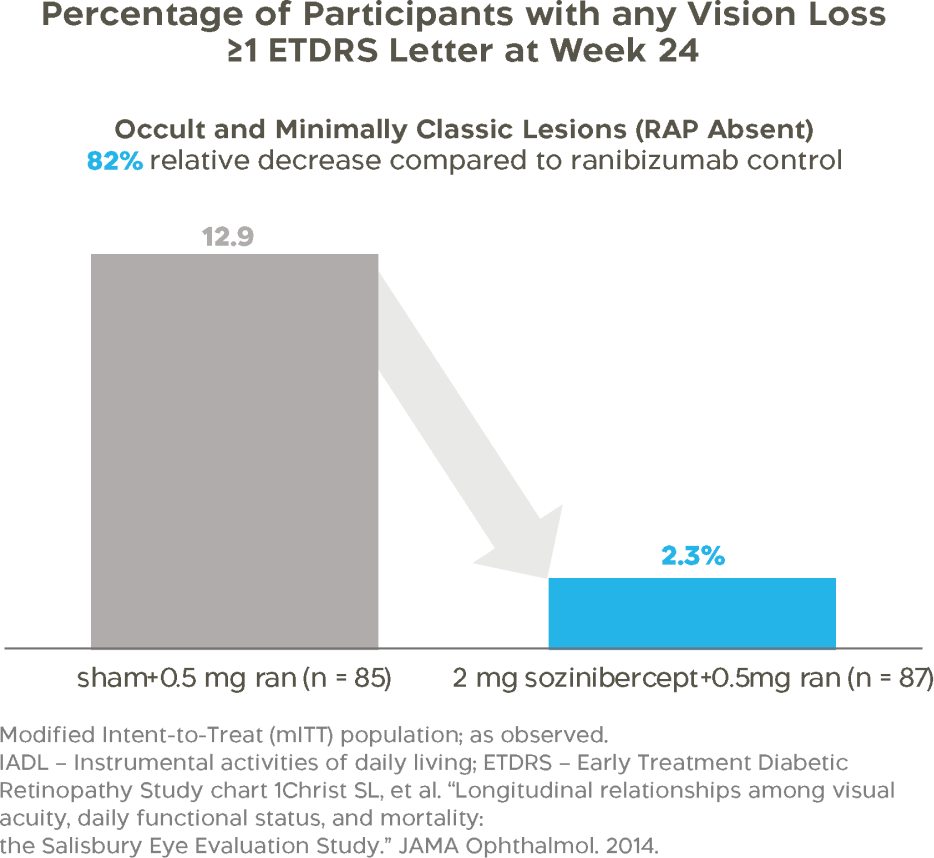
Compared to treatment with ranibizumab alone, combination therapy with sozinibercept reduced the proportion of patients experiencing vision loss by 82%.
Our Science Based on Bold Innovation
Sozinibercept combination therapy has the potential to elevate standard-of-care by delivering superior vision outcomes.


Explore publications relevant to sozinibercept, our clinical trials and the science behind our innovations.

